Contact Admission
International Collaboration
Updating the SOFA-2 Score: A New Standard in the Assessment of Multiple Organ Failure After Three Decades
The original SOFA score (SOFA-1), introduced in 1996, reflected the clinical practices of the 1990s. However, critical care medicine has advanced dramatically with the emergence of sophisticated life-support technologies, newer vasoactive agents, and a growing emphasis on minimally invasive interventions. Continued reliance on an outdated “measurement tool” may result in inaccurate assessment of disease severity and mortality risk in the context of modern medical practice. The SOFA-2 score was therefore updated to better reflect contemporary organ-support strategies and revised scoring thresholds, aiming to characterize organ dysfunction across a large population of critically ill patients with diverse pathologies, spanning different geographic regions and socio-economic settings.
Study Scope and Methodological Framework
The SOFA-2 study represents a large-scale international effort conducted across eight phases, analyzing data from 3.34 million patients treated in 1,319 ICU units across nine countries (Australia, Austria, Brazil, France, Italy, Japan, Nepal, New Zealand, and the United States) between 2014 and 2023. A panel of 60 critical care experts employed a modified Delphi (mDelphi) process to achieve consensus on appropriate clinical definitions and variables. The dataset was subsequently divided into cohorts for internal validation (over 2 million patients) and external validation (over 1.2 million patients), using advanced statistical modeling techniques, including generalized additive models (GAM) and classification and regression trees (CART).
Detailed Updates Across the Six Organ Systems of SOFA-2
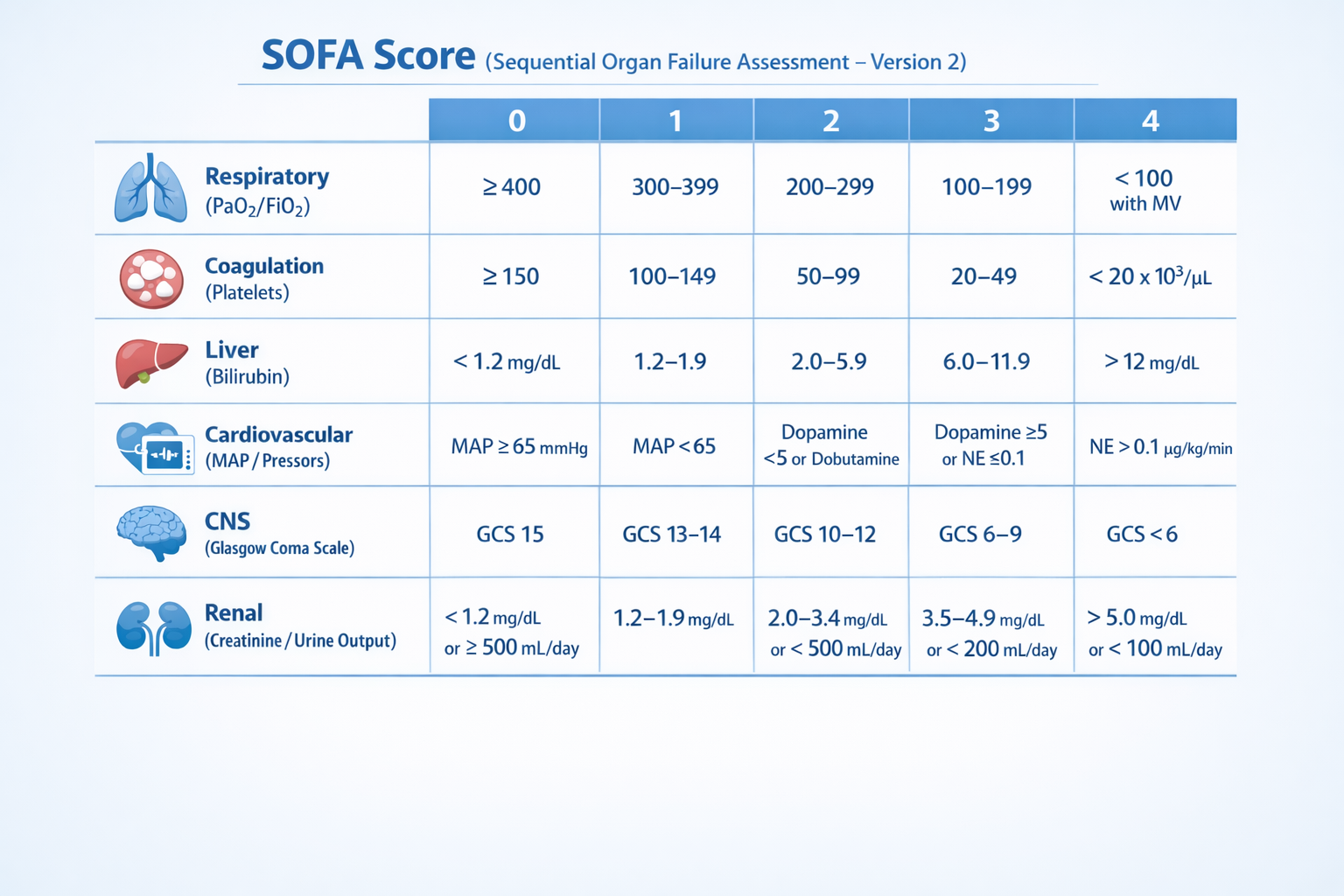
SOFA-2 retains the six-organ system structure with a total score ranging from 0 to 24; however, the scoring thresholds and clinical variables have been substantially refined.
For the neurological system, the Glasgow Coma Scale (GCS) is applied with the following categories: 15 (0 points), 13–14 (1 point), 9–12 (2 points), 6–8 (3 points), and 3–5 (4 points). In addition, patients requiring pharmacological treatment for delirium are assigned a minimum of 1 point, even when the GCS score is 15. In the respiratory system, the PaO₂/FiO₂ ratio has been recalibrated using updated thresholds: >300 (0 points), ≤300 (1 point), ≤225 (2 points), ≤150 (3 points), and ≤75 (4 points). Patients receiving ECMO for respiratory failure are assigned 4 points. When arterial blood gas analysis is unavailable, the SpO₂/FiO₂ ratio may be used as a surrogate, provided that SpO₂ is <98%. The cardiovascular system classifies vasoactive support based on the total dose of norepinephrine- and epinephrine-equivalent therapy, with thresholds of ≤0.2 µg/kg/min (2 points), >0.2 to ≤0.4 µg/kg/min (3 points), and >0.4 µg/kg/min (4 points). Mechanical circulatory support modalities, including IABP, LVAD, and VA-ECMO, are incorporated into the 4-point category.
For the hepatic system, updated total bilirubin thresholds are defined as ≤1.2 (0 points), ≤3.0 (1 point), ≤6.0 (2 points), ≤12.0 (3 points), and >12.0 mg/dL (4 points). The renal system is assessed using serum creatinine levels, urine output, or the need for renal replacement therapy (RRT); patients currently receiving RRT or meeting criteria for RRT are assigned 4 points. The coagulation system retains the original platelet count thresholds: >150 (0 points), ≤150 (1 point), ≤100 (2 points), ≤80 (3 points), and ≤50 × 10³/µL (4 points). Notably, the gastrointestinal and immune systems were considered during model development but were ultimately excluded due to insufficient predictive value or lack of data consistency.
Important Scoring Rules
On the first day in the ICU, missing data values are generally assigned 0 points; on subsequent days, the most recent previously observed value is carried forward. For sedated patients, the GCS score recorded immediately prior to sedation is used. Scores of 3 and 4 in the respiratory system are applied only when advanced respiratory support is in place, such as invasive or noninvasive mechanical ventilation, or high-flow nasal cannula (HFNC). For vasoactive agents, scoring is performed only if the drug is administered as a continuous intravenous infusion for at least 1 hour, and doses must be calculated using the base form (for norepinephrine).
Clinical Outcomes and Performance
SOFA-2 demonstrated a slightly superior ability to predict ICU mortality compared with SOFA-1, with AUROC values of 0.79 versus 0.77, respectively. Nearly 50% of patients were reclassified when transitioning from SOFA-1 to SOFA-2; the observed differences in mortality rates—13.5% when SOFA-2 scores were higher and 8.6% when they were lower than SOFA-1—suggest that the updated score more accurately reflects the severity of organ dysfunction in contemporary clinical practice. In addition, SOFA-2 maintained stable predictive performance from ICU day 1 through day 7 over the course of critical care treatment.
Limitations of the Study
The study used ICU mortality as the sole primary outcome for evaluation. The SOFA-2 score has been validated only in adult patients treated in the ICU, and therefore its applicability to pediatric populations or patients outside the intensive care setting cannot yet be established.
In summary, SOFA-2 represents more than a simple adjustment of numerical thresholds; it constitutes a comprehensive upgrade that integrates modern critical care technologies, enabling clinicians to achieve a more accurate, equitable, and context-appropriate assessment of patient status across diverse healthcare systems worldwide.
Read the full article in JAMA.
Other news
- How Dangerous Is Nipah Virus? Medical Alert and Urgent Health Recommendations ( 14:13 - 27/01/2026 )
- Predicting Disease from Sleep – A New Breakthrough Study ( 14:01 - 13/01/2026 )
- Medical advances predicted to break through in 2026 ( 13:54 - 12/01/2026 )
- Vietnamese medical miracles in 2025 – inspiration for medical students ( 07:54 - 07/01/2026 )
- Home AEDs: High Life-Saving Effectiveness, but Not Cost-Effective at Current Prices ( 14:12 - 18/12/2025 )
- Artificial Intelligence and Pediatric Care ( 08:27 - 16/12/2025 )
- Applying Clinical Licensing Principles to Artificial Intelligence ( 09:36 - 08/12/2025 )
- U.S. Approves Targeted Lung Cancer Therapy Datroway ( 08:43 - 25/06/2025 )
- Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing ( 08:38 - 23/11/2023 )
- Symbol of medicine ( 19:38 - 19/09/2021 )