Contact Admission
International Collaboration
Apnea in Preterm Infants and the MoCHA Trial
The MoCHA Randomized Clinical Trial
Waldemar A. Carlo, MD; Eric C. Eichenwald, MD; Benjamin A. Carper, MS
Minh Thuy Pham, MD, MSc
Introduction to Apnea in Preterm Infants and the MoCHA Study
Prolonged hospitalization in moderately preterm infants is often due to two main factors: persistent apnea and the inability to feed orally. Caffeine is a common and effective treatment for reducing apnea in preterm infants. However, caffeine use may lead to side effects, and there is currently a lack of clear evidence regarding the efficacy and safety of continuing caffeine therapy after apnea has improved. As a result, the optimal timing for discontinuing caffeine in preterm infants remains uncertain, leading to significant variations in clinical practice across hospitals.
Against this backdrop, the MoCHA (Moderately Preterm Infants Discharged With Caffeine at Home for Apnea) Randomized Clinical Trial was conducted. The study aimed to evaluate whether extending caffeine therapy could shorten the length of hospital stay in moderately preterm infants, and whether it could reduce the rate of hospital readmissions or emergency department visits after discharge.
MoCHA Trial Design
Design: This was a randomized, placebo-controlled clinical trial conducted at 29 hospitals across the United States.
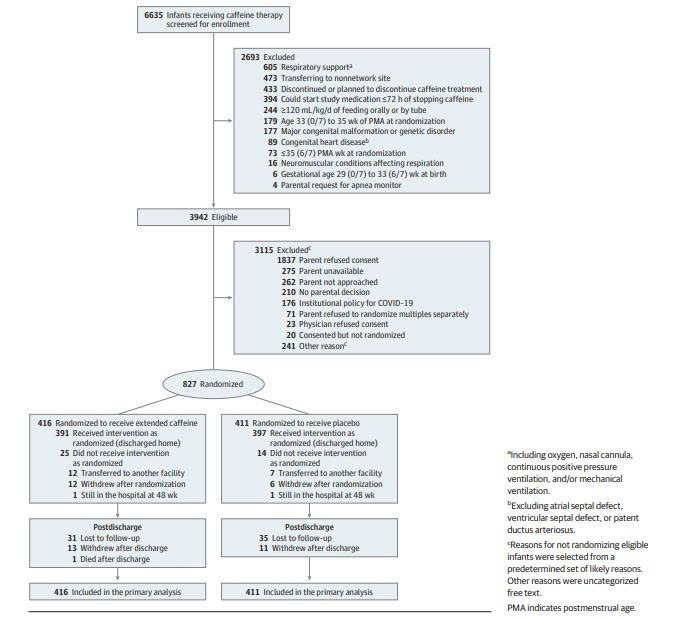
Participants: The study enrolled 827 moderately preterm infants born between 29 and 33 weeks of gestation. Specific inclusion criteria were as follows:
Postmenstrual age (PMA) between 33 weeks 0 days and 35 weeks 6 days at the time of randomization.
Currently receiving caffeine therapy with a plan to discontinue it.
Receiving oral or tube feedings totaling ≥120 mL/kg/day.
Ability to initiate the study drug within 72 hours of discontinuing caffeine.
Intervention: Infants were randomly assigned to one of two groups:
Caffeine group: Continued oral caffeine citrate at a dose of 10 mg/kg/day.
Placebo group: Received a caffeine-free solution.
The study drug was administered daily during hospitalization and continued for 28 days after discharge.
Primary Outcome: Number of days of hospitalization after randomization.
Secondary Outcomes: These included the number of days to achieve physiological maturity (defined as ≥5 consecutive days without apnea, full oral feeding, and ≥48 hours out of the incubator), postmenstrual age at discharge, number of hospital readmissions, number of emergency department visits or outpatient visits, and safety-related adverse events.
Primary Outcome and Key Findings
Total Enrollment: A total of 827 infants were randomized — 416 to the caffeine group and 411 to the placebo group.
No Reduction in Hospital Stay: The study found that extended caffeine therapy did not shorten the length of hospitalization after randomization. The median hospital stay was 18.0 days in the caffeine group versus 16.5 days in the placebo group.
Earlier Resolution of Apnea in the Caffeine Group: Although hospital stay was not reduced, infants in the caffeine group experienced resolution of apnea significantly earlier — a median of 6 days compared to 10 days in the placebo group.
Feeding Was the Main Discharge Determinant: There was no significant difference between groups in time to achieve physiological maturity or full oral feeding. This highlights that while caffeine improved apnea resolution, the ability to take full feeds orally remained the key factor determining discharge, not the resolution of apnea alone.
No Reduction in Readmissions or Medical Visits: Extended caffeine therapy did not reduce the rates of hospital readmissions or the number of emergency or outpatient visits after discharge.
Safety and Adverse Events:
There was no significant difference in the rate of serious adverse events between the two groups.
The caffeine group had a higher incidence of tachycardia (8.5% vs. 3.6%) and a lower discharge weight (2,585g vs. 2,659g), although these side effects were considered transient.
The caffeine group also had fewer clinically significant episodes of apnea or bradycardia after randomization (1.0% vs. 4.4% in the placebo group).
Limitations of the MoCHA Study
Early termination for futility: The trial was stopped early due to lack of benefit, which may have limited its ability to detect small but potentially meaningful differences between groups.
Lack of standardized apnea monitoring: Apnea events were assessed based on clinical documentation rather than objective, standardized monitoring tools, which may affect data accuracy.
Insufficient power for rare outcomes: The sample size was not large enough to reliably assess rare but serious adverse events such as seizures or post-discharge sudden infant death.
No long-term follow-up: The study did not evaluate the potential long-term effects of extended caffeine therapy on neurodevelopmental outcomes.
Conclusion and Clinical Implications
The MoCHA trial represents a significant advancement over previous studies, owing to its large sample size, multicenter design, and direct comparison of caffeine versus placebo in real-world clinical practice—allowing for clearer, more generalizable conclusions.
A key finding from this trial is that, in moderately preterm infants (born between 29 and 33 weeks of gestation) who are improving in terms of apnea, discontinuing caffeine prior to hospital discharge is safe and appropriate, unless specific indications warrant continued therapy.
This study reinforces the notion that the ability to achieve full oral feeding is the primary determinant of discharge readiness in preterm infants, rather than the complete resolution of all apnea episodes.