Contact Admission
International Collaboration
Scrotal Rash for Months
Winston W. Tan, MD; Matthew S. Soberano, DO; A. Toledo, DO; Matthew Clarence Tan
May 06, 2024
102
293
Add to Email Alerts
Editor's Note:
The Case Challenge series includes difficult-to-diagnose conditions, some of which are not frequently encountered by most clinicians, but are nonetheless important to accurately recognize. Test your diagnostic and treatment skills using the following patient scenario and corresponding questions. If you have a case that you would like to suggest for a future Case Challenge, please email us at ccsuggestions@medscape.com with the subject line "Case Challenge Suggestion." We look forward to hearing from you.
Background
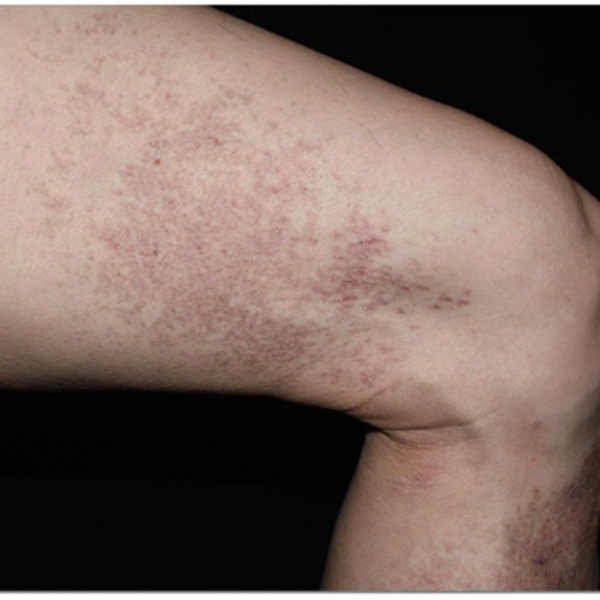
A 77-year-old man who was previously diagnosed with prostate cancer presents with a scrotal rash (such as the one illustrated in Figure 1).
Figure 1.
Approximately 10 years ago, a prostate-specific antigen (PSA) elevation of 15 ng/dL was detected. He had a biopsy that revealed prostate adenocarcinoma with high-grade intraepithelial neoplasia. He had a radical prostatectomy. Final pathology revealed a Gleason score of 4 + 4 = 8. The tumor was confined within the prostatic capsule. The seminal vesicle was negative for involvement, and the margins were free, making him stage T2NxMx.
The patient had undetectable PSA and no evidence of disease for more than a decade. When his PSA levels began to rise to 25 ng/dL, he was started on leuprolide; his PSA level became undetectable. Subsequently, he was placed on intermittent hormone treatment with leuprolide at 22.5 mg every 3 months if his PSA was above 5 ng/dL. Approximately 2 years ago, he developed bone metastasis to his sternum, spine, and pelvis. He became castrate resistant and also had congestive heart failure secondary to severe mitral regurgitation.
He presented for a consult due to congestive heart failure and a scrotal rash that had been present more than 3 months. The rash is erythematous, maculopapular, nonpruritic, and nontender. He has no palpable testicular mass. The patient's history is significant for genital edema secondary to congestive heart failure.
His family history is positive for congestive heart failure and coronary artery disease in his father and mother. He has a history of 30 pack-years of smoking, and he is married. He is also on the following medications:
-
Furosemide: 40 mg orally (PO) daily
-
Lisinopril: 10 mg PO daily
-
Aspirin: 325 mg/tab PO daily
-
Potassium chloride: 20 mEq daily
-
Leuprolide: 30 mg intramuscularly every 4 months
Physical Examination and Workup
The patient appears alert and oriented with no focal deficits and mild, acute distress. He is cooperative, with appropriate mood and affect. His blood pressure is 150/78 mm Hg. His heart rate is 103 beats/min. His respiratory rate is 22 breaths/min. His temperature is 98°F (36.7°C). His weight is 187.4 lbs (85 kg).
His pupils are equal, round, and reactive to light with normal conjunctiva. He has a prominent jugular venous pulse. His oral mucosa is moist, with no pharyngeal erythema. His neck is supple without carotid bruit. His breath is nonlabored, and the breath sounds are equal. He has symmetrical chest wall expansion, with faint crackles in the lower lobe. His cardiovascular findings reveal normal peripheral perfusion with no obvious carotid bruit heard bilaterally. He has a 4/6 systolic murmur in the midchest.
His abdomen is soft, nontender, and nondistended, with normal bowel sounds. He reports no diarrhea or constipation. He has grade 2 edema and a slightly unsteady gait when standing and uses a cane. The rash on the scrotum is maculopapular and on the right side. It has no exudate, is nontender, and does not have ulceration.
His PSA level is 155 ng/dL. His complete blood count is normal. His alkaline phosphatase level is 238 U/L (reference range, 44-147 IU/L). Chest radiography reveals bilateral lung infiltrate consistent with congestive heart failure. He has no chest pain or shortness of breath. A bone scan reveals evidence of rib and spine metastasis with no bone pain.
Discussion
Prostate cancer is the most common cancer among men and is the second leading cause of death. Although it is a slow-growing cancer, 34,500 of the 270,000 men diagnosed each year die.[1] An estimated 12.5% of men in the United States will develop prostate cancer during their lifetime. Age, hereditary factors, nutrition, and certain environmental factors are primary risks of developing prostate cancer.
Prostate cancer develops when cell division and cell death rates cease being equal. This leads to tumor growth that is uncontrolled. After the initial transformation, additional mutations affect various genes, including the genes for p53 and retinoblastoma; this can lead to progression of the tumor and metastasis. Most prostate cancers are adenocarcinomas.[2] Roughly 4% of cases have transitional cell morphology. These are believed to arise from the urothelial lining of the prostatic urethra. The rare cases that have neuroendocrine morphology are thought to arise from the neuroendocrine stem cells that are normally present in the prostate or from aberrant differentiation programs during cell transformation. Squamous cell carcinomas are rare, constituting less than 1% of all prostate carcinomas. In many cases, prostate carcinomas with squamous differentiation arise after radiation or hormone treatment.
Of prostate cancer cases, most arise in the peripheral zone (70%), 15%-20% arise in the central zone, and 10%-15% arise in the transitional zone. Most prostate cancers are multifocal, with synchronous involvement of multiple zones of the prostate, which may be due to clonal and nonclonal tumors.
Upon the diagnosis of prostate cancer with no metastasis, treatment options include prostatectomy, radiation, and interstitial seed implant or observation (watchful waiting). This patient had a radical prostatectomy. Postsurgery, PSA is often undetectable if the patient is cured of the disease. However, elevation of the PSA is the first sign of recurrence. In patients with no metastasis, patients are often either monitored or "started on antitestosterone treatment to suppress or make the cancer dormant."
The bone is the most common site of prostate cancer metastases; of those patients who have metastatic disease, 85% of patients develop bone metastases. Bone metastasis is commonly observed in the spine, pelvis, lumbar spine, and femur. Changes are osteoblastic, osteolytic, or mixed.[1,3] At times, metastasis from the vertebral body to the epidural space causes epidural compression of the spinal cord.[4] Other findings include anemia, bone marrow suppression, and fractures.
Additionally, the lymph nodes are also a common site for metastasis. Rare sites of metastasis include the skin, subcutaneous tissue, gastrointestinal tract, and brain.[5,6] Distant metastases to cutaneous and subcutaneous locations are present in 2%-9% of malignant tumors.[7,8]
The patient in this case was diagnosed with metastatic cancer on the skin of the scrotum. A punch biopsy of the skin revealed findings consistent with metastatic prostate cancer (Figure 2). PSA staining of the tissue was positive; this confirmed the prostate cancer as the origin of the metastasis (Figure 3).
Figure 2.
Figure 3.
The patient underwent mitral valve repair. During surgery, he was found to have metastasis to the lungs and the sternal bone. He recovered from surgery and slowly improved. He declined treatment except for leuprolide for the prostate cancer. His disease progressed, and he died within 6 months.
In a study of 2369 cases of cutaneous metastases arising from 81,618 primary solid visceral malignancies, skin metastasis was reported in 2.9%.[9] Primary urologic malignancies of the testes, prostate, bladder, and kidney were found in 116 (1.3%) of 10,417 cases. The incidence of cutaneous metastases from the testes, prostate, bladder, and kidney was 0.4%, 0.36%, 0.84%, and 3.4%, respectively. Overall, 436 cases of cutaneous metastases from urologic organs were catalogued. Infiltrated plaque or nodules were the most common presentations. Typically, the clinical features mimicked common skin disorders. From the date of presentation, disease-specific survival had a median of less than 6 months.
Cutaneous metastasis due to prostate cancer is extremely rare. In a review of pathologic specimens at UCLA Hospital, 2 of 136,017 surgical and postmortem specimens had this condition.[4] In another review, cutaneous metastases accounted for 0.3% of prostate metastases.[5] The lesions are often multiple solid nodules that rarely ulcerate. Occasionally, they present as red macules or papules, few in number, and are usually localized in the suprapubic and anterior aspect of the thigh. Cutaneous metastasis often signifies advanced disease and portends poor prognosis (most patients die within 6 months). Immunohistochemical study with PSA confirms the prostatic origin of metastases.
This patient presented with skin metastases 14 years after the initial diagnosis of prostate cancer. Recognizing cutaneous metastasis as a differential diagnosis of a skin lesion in a patient with a history of prostate cancer is important for both diagnostic and prognostic purposes.
When leuprolide stops working, several new drugs have been approved for patients with castration-resistant metastatic prostate cancer, including docetaxel, sipuleucel-T, abiraterone acetate with prednisone, and enzalutamide. All these have been approved by the US Food and Drug Administration (FDA) because they improve survival by 4-6 months, on average.[10]
With the exception of skin cancer, prostatic adenocarcinoma represents the most common cancer among men in the United States and is the second most common cause of cancer mortality. Mortality is often associated with metastatic disease; in the case of prostatic adenocarcinoma, this typically involves bones and rarely affects the skin. Although clinical history and examination, laboratory tests, and routine pathology can suggest the prostate as a source of metastatic disease, immunohistochemistry—specifically PSA—is often used to help establish the diagnosis.
Medscape © 2024 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Winston W. Tan, Matthew S. Soberano, A. Toledo, et. al. Scrotal Rash for Months - Medscape - May 06, 2024.
Other Case Report
- Challenges in Clinical Electrocardiography – A Mysterious Case of ST Elevation ( 10:29 - 15/09/2025 )
- New Secrets About Psoriatic Arthritis: Is Combination Therapy as Safe as You Think? ( 14:33 - 06/09/2025 )
- Mitrofanoff procedure: more 40 years later. ( 07:36 - 28/03/2025 )
- Telehealth vs In-Person Early Palliative Care for Patients With Advanced Lung Cancer ( 09:26 - 12/10/2024 )
- ST-Segment Elevation in a Woman With Out-of-Hospital Cardiac Arrest ( 08:35 - 24/09/2024 )
- Lower Gastrointestinal Hemorrhage ( 16:15 - 16/05/2024 )
- Can Cardiovascular Risk Assessment Be Improved in the 21st Century? ( 15:57 - 16/05/2024 )
- Approach to Obesity Treatment in Primary Care ( 14:04 - 20/03/2024 )
- Fever, Rash, and Shortness of Breath in a 69-Year-Old ( 09:20 - 04/03/2024 )