Contact Admission
International Collaboration
Mitrofanoff Surgery: A 40-Year Retrospective
Nguyễn Hữu Phùng1,2, Nguyễn Tăng Miên3, Nguyễn Quang Tập3, Bùi Chún3
1 Tam Tri Da Nang General Hospital
2 Phan Chau Trinh University
3 Da Nang General Hospital
Since 1982, Da Nang Hospital has implemented the Mitrofanoff procedure — a surgical technique that creates a continent urinary conduit using the appendix, allowing patients to control urinary excretion. Over more than 40 years, the hospital has conducted long-term follow-ups on four pediatric cases, with an average follow-up duration of 32 years (ranging from 23 to 42 years).
I. Introduction
Mitrofanoff’s idea of using an appendix with an intact vascular pedicle to create a continent catheterizable channel for bladder drainage in patients with neurogenic bladder was both innovative and deeply humane.
The core significance of this technique lies in enabling patients to control their urinary flow—preventing involuntary leakage and allowing urine to be evacuated at will through catheterization, thanks to the flap-valve mechanism. Post-surgery, patients no longer suffer from the unpleasant odor of urine, enabling them to confidently participate in social life. Originally designed for neurogenic bladder, the Mitrofanoff principle has gradually been applied to various other urological conditions [8,10,18], and later inspired a broader concept of continent urinary diversion that Rachet Barat [1] described as a revolution in urinary diversion surgery.
We first applied this technique at Da Nang General Hospital in early 1982 on a 12-year-old patient. Since then, we have performed 10 cases with different underlying pathologies, including 4 pediatric patients.
Objective: To evaluate the long-term outcomes in four pediatric patients who underwent Mitrofanoff surgery, as well as their quality of life over an average follow-up period of 32 years (ranging from 23 to 42 years).
II. Surgical Case Presentation:
From early 1982 to 2001, we performed four pediatric cases following the Mitrofanoff principle.
| Year of Surgery |
|
Age at Surgery (years) |
|---|---|---|
| Case 1 – 1982 | Cystectomy + Replacement with Ileal Conduit | 12 |
| Case 2 – 1990 | Bladder Augmentation – Ileal Patch | 9 |
| Case 3 – 1998 | Bladder Augmentation – Gastric Patch | 6 |
| Case 4 – 2001 | Bladder Augmentation – Gastric Patch |
4 |
Table 1. Bladder Augmentation Techniques Combined with Mitrofanoff Procedure

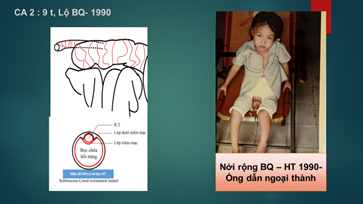
In two cases, we constructed the Mitrofanoff conduit as follows:
A 3 cm longitudinal incision was made through the seromuscular layer of the ileal segment to expose the submucosa. The appendix was then inserted into this space, and a small opening was created in the ileal mucosa to anastomose the beveled end of the appendix to the ileal mucosa. Subsequently, the seromuscular layer of the ileum was sutured over the appendix for a length of 3–4 cm, creating a submucosa-lined extramural tunnel (Figure 2).
| No | Technique for Creating Conduit Tunnel |
|---|---|
| 1 | Intramucosal bladder tunnel |
| 2 | Submucosa-lined extramural tunnel: ileum – Submucosa-lined extramural tunnel |
Table 2. Techniques for Creating Anti-Reflux Valves
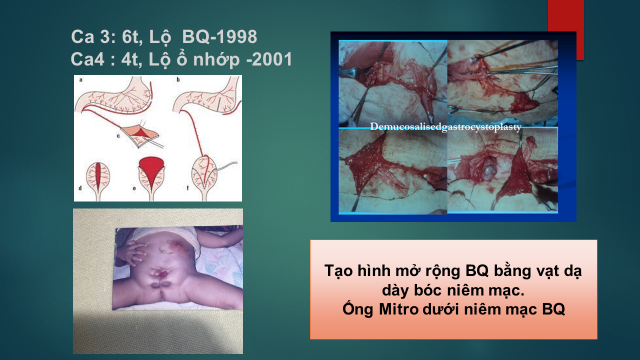
Figure 3. Creation of a Gastric Seromuscular Flap to the Bladder While Preserving Bladder Mucosa
Clean Intermittent Catheterization (CIC) After Surgery:
None of the patients had prior experience with clean intermittent catheterization (CIC) through the natural urethral route before undergoing surgery. The commonly used catheter was the inexpensive plastic Nelaton catheter, selected in sizes appropriate to the Mitrofanoff conduit. Patients were instructed to perform catheterization 5–6 times daily using 5–6 sterile Nelaton catheters.
In younger patients, catheterization was performed by their parents. After each use, the catheter was rinsed, placed in a tightly tied plastic bag, and boiled for 30 minutes for reuse throughout the day. Any catheter that became rigid was discarded.
Most patients developed awareness and adherence to the CIC routine. One patient, due to work constraints, occasionally left the catheter in place during the day instead of performing CIC.
Another patient retained an indwelling catheter for over 15 years due to conduit incontinence through the Mitrofanoff channel, until a corrective surgery was eventually performed.
III. Results
There were no postoperative deaths.
3.1 Complications and Management Approaches
There were four types of surgical complications observed across all four patients, resulting in a total of eight surgical interventions.
Each of the four patients experienced a different surgical complication. One patient had bladder stones that did not require surgery, one underwent a single surgical intervention, another required two surgeries, and one patient underwent five procedures.
Out of the eight total surgeries, two were performed within the first four years postoperatively, while the remaining six were carried out in the subsequent years.
3.1.1 Stones
Stones were the most common complication, occurring in 75% of patients. Three patients with augmented bladders developed large, soft bladder stones. Among them, one patient (Case 2) with bladder exstrophy anastomosed to an ileal segment had both bladder stones and unilateral kidney stones. One patient (Case 3) underwent three separate open surgeries for stone removal. All cases required open surgical stone extraction.
In contrast, one patient (Case 1) with an intact ileal reservoir experienced recurrent small stones (0.3–0.6 cm in diameter) that were regularly expelled through the Mitrofanoff conduit during catheterization, without requiring surgery over a 42-year period.
3.1.2 Incontinence of the Mitrofanoff Conduit
Incontinence of the Mitrofanoff Conduit
This complication occurred in Cases 3 and 4—one patient with bladder exstrophy and the other with a cloacal exstrophy. Both underwent reconstruction using demucosalized gastrocystoplasty with preservation of the native bladder urothelium. Bladder fistula and conduit incontinence developed some time after surgery.
One patient required surgical revision of the Mitrofanoff conduit 4 years postoperatively. The other patient underwent revision 18 years after the initial procedure. In this latter case, in addition to incontinence, the conduit was also stenotic and short, measuring only 3 cm. The surgeon (Dr. Roberto de Castro) had to excise the original conduit and replace it with a Monti tube in 2016. Both patients now have continent Mitrofanoff conduits.
3.1.3 Postoperative Bladder Fistula Bladder fistulas developed postoperatively in two patients (Cases 3 and 4).
The likely cause was inadequate adherence of the gastric seromuscular flap to the bladder wall due to flap contraction. These flaps were eventually excised and replaced with traditional ileal patches after 2 and 4 years, respectively.
3.1.4 Reservoir-to-Ureteral Reflux: Case 1
Reservoir-to-Ureteral Reflux: Case 1
This patient lost both the bladder and urethra due to a war injury and underwent cystectomy with replacement using an intact ileal segment in 1982. The ureters were implanted directly into the ileal reservoir. A grade 3 vesicoureteral reflux was detected 15 years later. As of now—40 years postoperatively—the reflux persists, and the patient shows signs of chronic renal failure.
Reservoir pressure in Case 1: 60 cm H₂O
Reservoir pressure in Case 4: 10 cm H₂O
3.2. Current Occupation:
-
Case 1: Spray painter, father of two children
-
Case 2: Hairdresser
-
Case 3: Mobile phone repair technician and online seller
-
Case 4: Small business owner and active in charity work
IV. Discussion
Complication Issues:
Following Mitrofanoff surgery, nearly all patients face potential complications, which may arise at any time postoperatively. These include bladder (reservoir) stones, bladder perforation, bowel obstruction, and conduit incontinence, as reported in previous studies [18, 24, 26, 6, 19]. In the study by Liard et al. [18], which followed 22 patients over 20 years, 36 complications were recorded, requiring 31 surgical interventions.
In our series, we observed a notably high complication rate of 8 complications in 4 patients, with a similarly high rate of reoperations (8 surgeries in 4 patients).
These complications stem from two distinct sources:
-
Complications related to the urinary reservoir
-
Complications related to the Mitrofanoff conduit
1. Reservoir-to-ureterorenal reflux occurred in 1 out of 4 cases.
In Case 1, the urinary reservoir was created from an intact ileal segment, which was not detubularized. As a result, the pressure remained high. After more than 42 years of follow-up, we measured the reservoir pressure and found it to be as high as 60 cm H₂O. In contrast, Case 4, who underwent bladder augmentation with a detubularized intestinal segment, showed a much lower reservoir pressure of only 10 cm H₂O.
Romero-Maroto J et al. [24] reported that in 19 patients with neurogenic bladder who underwent bladder augmentation to create a low-pressure reservoir and were followed for an average of 20 years, there was a significant reduction in vesicoureteral reflux and hydronephrosis. Liard and Mitrofanoff [18] reported on 23 patients operated according to the Mitrofanoff principle, with 10 of them developing vesicoureteral reflux due to preservation of the native neurogenic bladder. Among these, 6 required subsequent bladder augmentation to manage the reflux, and 4 were converted to permanent incontinent diversion.
Although bladder augmentation has been practiced for over a century [13], the concept of a low-pressure reservoir was only introduced in the 1980s [21, 22]. At the time of our first case in 1982, due to the challenging conditions in our country, we were unaware of this concept.
Creating a low-pressure reservoir should be considered the first essential principle for achieving successful outcomes in Mitrofanoff surgery.
2. Bladder stones are the most common surgical complication in bladder-intestinal reconstruction with a concomitant Mitrofanoff procedure.
The incidence of bladder stones can reach up to 52.5% [6], and the recurrence rate may be as high as 50% within 5 years [12].
Bruce Blyth et al. [2] found that bladder stones occurred in 30% of patients, and 90% of those cases developed an average of 25 months after surgery.
We do not know exactly how long after surgery bladder or reservoir stones developed in our patients, but in our report, the incidence of stone-related complications was 75%, with a recurrence rate of 33.3%. Stones accounted for 62.5% of all complications during the 32-year follow-up period.
The cause of stone formation remains a subject of debate. It may be due to urinary tract infections, incomplete emptying of the reservoir during each CIC session, irregular or noncompliant CIC routines, or metabolic disorders [27,16,9].
Stones may be removed either via open surgery or endoscopic techniques [26], but daily irrigation of the reservoir with saline is a simple preventive measure that may reduce recurrence rates [22].
The high incidence of stone-related complications in our report may be attributed to patients not fully adhering to medical guidance.
Notably, in the case involving an intact ileal conduit reservoir (case 1), the presence of peristalsis and high reservoir pressure resulted in vesicoureteral reflux and kidney damage. However, it did not lead to the formation of large calculi. Instead, small stones (3–6 mm in diameter) were consistently passed through the Mitrofanoff channel during CIC. To the best of our knowledge, no similar case has been reported in the medical literature to date.
3. Cutaneous Vesicostomy Fistula
Both cases of bladder fistula in this report were associated with demucosalized gastric flaps, which appear to have increased the rate of postoperative complications. The failure of bladder augmentation using demucosalized gastric flaps while preserving the native bladder urothelium has also been reported in previous studies [3, 4, 25], indicating that this technique remains unreliable despite its theoretical advantages.
This failure suggests that ileal bladder augmentation should be the preferred method.
4.Complications related to the Mitrofanoff conduit:
Assessment of Successful Mitrofanoff Surgery:
A successful Mitrofanoff procedure is defined by the continence of the Mitrofanoff channel, i.e., its ability to control urinary leakage. In our report, two patients had Mitrofanoff conduits tunneled beneath the bladder mucosa which later became incontinent and had to be removed and replaced with Monti channels. After surgical revision, both patients have maintained good urinary continence to date.
We would also like to emphasize that the two submucosa-lined extramural ileal tunnels (i.e., tunneled outside the intestinal mucosa) continue to function well postoperatively.
The reported success rates for Mitrofanoff continence vary among authors—from 100% (Duckett [7]), 73.5% (Lefevre [19]), to as low as 50%. Liard et al. [18] followed 23 patients over 20 years and found that 11 cases (50%) developed channel complications such as stenosis or incontinence that required surgical revision. Ultimately, 79% achieved continence, but one patient failed all interventions and was eventually diverted to a non-continent urinary diversion, which is considered a major surgical failure.
Hampson, L. et al. [9] noted that in pediatric patients, Mitrofanoff surgery must account for growth and abdominal wall development. As the child grows, the bladder structure may not expand proportionally with the abdominal wall, causing stretching of the appendix, which may impair the continence of the channel.
Persistent Mitrofanoff incontinence despite multiple revisions, necessitating permanent incontinent diversion, remains an undesirable outcome.
Postoperative Life: All patients in our study maintain relatively stable, independent lives. One female patient suffers from depression but continues to make a living as a hairdresser.
V. Conclusion
We report four cases of surgery based on the Mitrofanoff principle, performed at Da Nang Hospital between 1982 and 2001, in patients aged 4 to 12 years old. These cases have been followed for an average of over 32 years.
No postoperative deaths were recorded throughout the follow-up period.
The rate of complications and reoperations was high, mainly related to bladder stones and Mitrofanoff conduit continence. In one case, the intestinal segment used for urinary diversion was not detubularised, resulting in high reservoir pressure and severe ureterohydronephrosis due to reflux. In two cases, bladder augmentation using demucosalised gastric flaps while preserving native bladder urothelium led to undesirable outcomes and contributed to the overall complication rate.
However, all complications were eventually managed, and all patients are currently leading stable, independent lives.
This is a complex surgical procedure with many potential long-term complications. Surgeons and patients should carefully consider the indications before surgery. Long-term follow-up is essential for all patients after surgery.
Bibliography
-
-
Barratt, R., Marsden, T., & Greenwell, T. J. (2018). The Mitrofanoff procedure: A continent revolution. Urology News, 22(2).
-
Blyth, B., Ewalt, D. H., Duckett, J. W., & Snyder, H. M. (1992). Lithogenic properties of enterocystoplasty. Journal of Urology, 148(4), 575–577.
-
Buson, H., Manivel, J. AC., et al. (1994). Seromuscular colocystoplasty lined with urothelium: Experimental study. Urology, 44(5), 743–748. https://doi.org/10.1016/s0090-4295(94)80220-3
-
Carr, M. C., Docimo, S. G., & Mitchell, M. E. (1999). Bladder augmentation with urothelial preservation. Journal of Urology, 162(3), 1133–1136. https://doi.org/10.1016/S0022-5347(01)68097-2
-
Castellan, M., Gosalbez, R., et al. (2012). Complications after use of gastric segments for lower urinary tract reconstruction. Journal of Urology, 187(5), 1823–1827. https://doi.org/10.1016/j.juro.2011.12.072
-
DeFoor, W., Minevich, E., et al. (2004). Bladder calculi after augmentation cystoplasty: Risk factors and prevention strategies. Journal of Urology, 172(5 Pt 1), 1964–196
-
Duckett, J. W., & Lotfi, A. H. (1993). Appendicovesicostomy (and variations) in bladder reconstruction. Journal of Urology, 149(3), 567–569. https://doi.org/10.1016/s0022-5347(17)36150-5
-
Gowda, B. D. R., Agrawal, V., & Harrison, S. C. W. (2008). The continent, catheterizable abdominal conduit in adult urological practice. BJU International, 102(11), 1688–1692.
-
Hampson, L. A., Baradaran, N., & Elliot, S. P. (2018). Long-term complications of continent catheterizable channels: A problem for transitional urologists. Translational Andrology and Urology, 7(4), 558–566.
-
Harris, C. F., Cooper, C. S., et al. (2000). Appendicovesicostomy: The Mitrofanoff procedure – A 15-year perspective. Journal of Urology, 163(6), 1922–1926.
-
Hensle, T. W., Kirsch, A. J., et al. (2004). Bladder neck closure in association with continent urinary diversion.
-
Husmann, D. A. (2009). Malignancy after gastrointestinal augmentation in childhood. Therapeutic Advances in Urology, 1(1), 5–11. https://doi.org/10.1177/1756287209104163
-
Jednak, R. (2014). The evolution of bladder augmentation: From creating a reservoir to reconstituting an organ. Frontiers in Pediatrics, 2, 10.
-
Kavanagh, A., Afshar, K., et al. (2012). Bladder neck closure in conjunction with enterocystoplasty and Mitrofanoff diversion for complex incontinence: Closing the door for good. Journal of Urology, 188(4 Suppl), 1561–1565. https://doi.org/10.1016/j.juro.2012.02.027
-
Khoury, A. E., Salomon, M., et al. (1997). Stone formation after augmentation cystoplasty: The role of intestinal mucus. Journal of Urology, 158(3 Pt 2), 1133–1137. https://doi.org/10.1016/S0022-5347(01)68097-2
-
Kreder, K. J., & Webster, G. D. (1992). Management of the bladder outlet in patients requiring enterocystoplasty. Journal of Urology, 147(1), 38–41.
-
Kurzrock, E. A., Baskin, L. S., & Kogan, B. A. (2012). Gastrocystoplasty: Long-term follow-up.
-
Liard, A., Seguier-Lipszyc, E., et al. (2001). The Mitrofanoff procedure: 20 years later. Journal of Urology, 165(6 Pt 2), 2394–2397.
-
Lefèvre, M., Faraj, S., et al. Appendicovesicostomy (Mitrofanoff procedure) in children: Long-term follow-up and specific complications.
-
Michaud, J., Jayman, J., & Gearhart, J. (2018). Stone formation and bacteriology after bladder augmentation in bladder exstrophy. Society for Pediatric Urology Fall Congress. Johns Hopkins School of Medicine, Baltimore, MD, USA.
-
Mitchell, M. E. (1981). The role of bladder augmentation in undiversion. Journal of Pediatric Surgery, 16, 790–794.
-
Mitchell, M. E., Kulb, T. B., & Backes, D. J. (1986). Intestinoplasty in combination with clean intermittent catheterization in the management of vesical dysfunction. Journal of Urology, 136, 288–292.
-
Mitrofanoff, P. (1980). Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chirurgie Pédiatrique, 21(4), 297–305.
-
Romero-Maroto, J., Martinez-Cayuelas, L., et al. (2021). Long-term effectiveness and safety of bladder augmentation in spina bifida patients. Neurourology and Urodynamics, 40(6), 1576–1584. https://doi.org/10.1002/nau.24713
-
Sun, X. G., Li, Y. X., et al. (2022). Outcomes of seromuscular bladder augmentation compared with standard bladder augmentation in the treatment of children with neurogenic bladder. World Journal of Clinical Cases, 10(23), 8115–8123. https://doi.org/10.12998/wjcc.v10.i23.8115
-
Szymanski, K. M., Misseri, R., et al. (2014). Additional surgeries after bladder augmentation in patients with spina bifida in the 21st century.
-
Szymanski, K. M., Misseri, R., et al. (2014). Cutting for stone in augmented bladders—What is the risk of recurrence and is it impacted by treatment modality? Journal of Urology: Pediatric Urology.
-
Other research
- Feminizing Gender-Affirming Hormone Therapy (fGAHT): A Comprehensive Medical Care Guideline ( 14:09 - 28/07/2025 )
- Cancer - Targeted treatments and navigation tests ( 10:42 - 08/09/2020 )
- Cancer - Targeted treatments and navigation tests ( 10:36 - 08/09/2020 )
- Microbiological agents cause hospital pneumonia ( 10:32 - 08/09/2020 )
- Cancer - Causes and Prevention ( 10:31 - 08/09/2020 )
- Survey on clinical characteristics and relationship with dengue virus type in dengue patients admitted to Quang Nam Regional General Hospital during the 2018 outbreak ( 15:24 - 06/04/2019 )