Contact Admission
International Collaboration
Inhaler Switching: Do Environmental Benefits Come at the Expense of Patient Health in COPD and Asthma Treatment
A large-scale study from the U.S. Veterans Health Administration (VHA) evaluated the impact of switching inhaler medications used to treat COPD and asthma. The study found that switching from a budesonide-formoterol metered-dose inhaler (MDI) to a fluticasone-salmeterol dry powder inhaler (DPI), while potentially reducing greenhouse gas emissions, was associated with increased healthcare utilization and adverse health events among patients. This raises important questions about the balance between environmental sustainability goals and clinical outcomes for patients.
To learn more about this study, you can read the full translated article from JAMA Internal Medicine here.
1. Disease Overview
Chronic obstructive pulmonary disease (COPD) and asthma are common chronic respiratory conditions that affect millions of people worldwide. Treatment typically involves the use of combination inhalers containing inhaled corticosteroids (ICS) and long-acting β-agonists (LABAs) to control symptoms and prevent exacerbations.
Currently, there are two main types of inhaler devices used to deliver medication to the lungs:
-
Metered-dose inhalers (MDIs): These devices use hydrofluorocarbon (HFC) propellants to deliver medication to the lungs. HFCs are potent greenhouse gases, with a global warming potential thousands of times greater than carbon dioxide. MDIs deliver medication independent of the patient’s inhalation effort but require coordination between actuation and inhalation.
-
Dry powder inhalers (DPIs): These inhalers do not use propellants and are considered more environmentally friendly. However, they require patients to inhale quickly and deeply to ensure the medication reaches the lungs effectively—something that may be challenging for individuals with impaired lung function.
To reduce greenhouse gas emissions in healthcare, several global health organizations—including the UK’s National Health Service (NHS), the Canadian Thoracic Society, and the National Asthma Council Australia—have recommended switching patients from MDIs to DPIs when clinically appropriate. However, this shift presents clinical challenges, as medications within the same therapeutic class can differ in active ingredients and device characteristics.
2. New Findings
In July 2021, the U.S. Veterans Health Administration (VHA) implemented a major change in its inhaler formulary following a cost-based competitive bidding process. The metered-dose inhaler budesonide-formoterol (Symbicort) was replaced with the dry powder inhaler fluticasone-salmeterol (Wixela Inhub) as the preferred option for patients with COPD and asthma.
This study, based on data from the U.S. VA healthcare system between January 2018 and December 2022, evaluated differences in clinical outcomes following this formulary change. It included two main analyses:
Self-Controlled Case Series (SCCS): Tracked 260,268 patients who switched inhalers. The SCCS cohort had a median age of 71 years, with 91% male, most diagnosed with COPD (69%), and 81% having a history of smoking.
Observational Cohort Study: Compared 167,331 patients who switched medications with 91,226 patients who did not.
Key Findings:
Increased healthcare utilization and adverse events (SCCS results):
In the Self-Controlled Case Series (SCCS), the use of dry powder fluticasone-salmeterol was associated with:
A 10% decrease in albuterol (rescue inhaler) dispensations.
A 2% increase in prednisone (rescue steroid) dispensations.
A 5% increase in all-cause emergency department visits.
An 8% increase in all-cause hospitalizations.
A 10% increase in respiratory-related hospitalizations.
A 24% increase in hospitalizations due to pneumonia.
Notably, among the 16,855 patients hospitalized for pneumonia during the study period, the pneumonia hospitalization rate was 24% higher with fluticasone-salmeterol use compared to budesonide-formoterol.
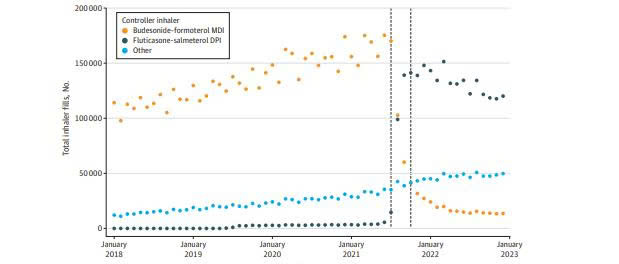
Findings from the observational cohort study:
There was no difference in mortality between the switched and non-switched groups after 180 days.
There were no significant changes in the use of supplemental albuterol or prednisone after 180 days (in contrast to the SCCS results).
However, patients who switched had increased rates of all-cause hospitalizations (16.14% vs. 15.64%), respiratory-related hospitalizations (3.15% vs. 2.74%), and pneumonia-related hospitalizations (1.15% vs. 1.03%) after 180 days.
This translates to an estimated 310 additional pneumonia-related hospitalizations if all 258,557 patients in the analysis had switched inhalers.
Environmental benefit:
The formulary change significantly reduced greenhouse gas emissions associated with inhaler use.
The average carbon dioxide equivalent emissions per veteran in the SCCS group decreased from 279.8 kg CO₂e in 2018 to 101.0 kg CO₂e in 2022.
This reduction is estimated to be equivalent to removing approximately 6,000 gasoline-powered passenger vehicles from the road for one year.
3. Clinical Implications
These findings suggest that switching from metered-dose inhalers (MDIs) containing budesonide-formoterol to dry powder inhalers (DPIs) containing fluticasone-salmeterol within the VHA system was associated with increased healthcare utilization and potential clinical harm to patients. Several factors may explain this:
Pharmacological differences between medications:
-
Fluticasone may be associated with a higher risk of pneumonia compared to budesonide, possibly due to its longer-lasting systemic and local immunosuppressive effects.
-
Salmeterol (a long-acting beta agonist, or LABA) has a slower onset of action than formoterol, which could negatively affect patients’ perception of inhaler effectiveness—particularly important in asthma management where formoterol-containing combinations are generally preferred.
Differences in delivery devices:
-
Dry powder inhalers (DPIs) require patients to perform a fast and deep inhalation, which can be difficult for older individuals or those with impaired lung function.
-
Lack of familiarity with the new device may lead to suboptimal drug delivery and poor disease control.
Treatment disruption:
-
Mandatory formulary changes may disrupt established treatment routines, leading to decreased medication adherence, inhaler technique errors, and worsening clinical outcomes.
-
A separate 2016 study found worse lung function outcomes in patients who were switched due to payer-driven formulary changes.
This study highlights the tension between cost containment, improved clinical outcomes, and environmental sustainability. While switching inhalers may reduce direct costs and greenhouse gas emissions, the increase in healthcare utilization—such as emergency visits and hospitalizations—may offset those climate benefits due to the high financial and resource burden of such services.
4. Recommendations
These findings underscore the need for more thoughtful implementation of large-scale inhaler switching policies:
Patient education and clear communication: Structured education programs on inhaler technique and clear communication between patients and healthcare providers during formulary transitions are essential to minimize care disruption.
Regular inhaler technique assessments: Ongoing evaluations of inhaler technique and peak flow monitoring can help improve drug delivery among patients switched to dry powder inhalers (DPIs).
Further research needed: Additional studies are necessary to refine inhaler-switching strategies across large healthcare systems in a way that optimizes both patient outcomes and global health objectives.
Balancing competing priorities: For healthcare systems aiming to reduce carbon emissions, interventions must strive to optimize clinical outcomes and net greenhouse gas reductions, while also maintaining cost-effectiveness.
Read the full study on the JAMA Internal Medicine website.
Other library
- How a Simple Urine Test Could Reveal Early-Stage Lung Cancer ( 10:08 - 26/01/2024 )
- "Allogeneic Stem Cell Therapy for Acute Ischemic Stroke The Phase 2/3 TREASURE Randomized Clinical Trial" ( 08:43 - 19/01/2024 )
- Can Endos Use Social Media Safely? ( 08:01 - 11/12/2023 )
- One in 10 People Who Had Omicron Got Long COVID: Study ( 20:25 - 01/06/2023 )
- Physical Medicine Academy Issues Guidance on Long COVID Neurologic Symptoms ( 09:58 - 19/05/2023 )
- Breakthrough' Study: Diabetes Drug Helps Prevent Long COVID ( 08:55 - 15/03/2023 )
- BCG vaccine (thuốc chủng ngừa bệnh lao) & SARS-CoV 2 (covid-19) infection ( 10:08 - 27/10/2022 )
- Đại dịch COVID-19 đã kết thúc? ( 09:11 - 22/09/2022 )
- Dị hình giới tính ở COVID-19: Ý nghĩa tiềm năng về lâm sàng và sức khỏe cộng đồng ( 09:22 - 19/03/2022 )
- COVID-19 Update ( 21:00 - 06/03/2022 )
















