Contact Admission
International Collaboration
Device pain relief
Pain relief equipment
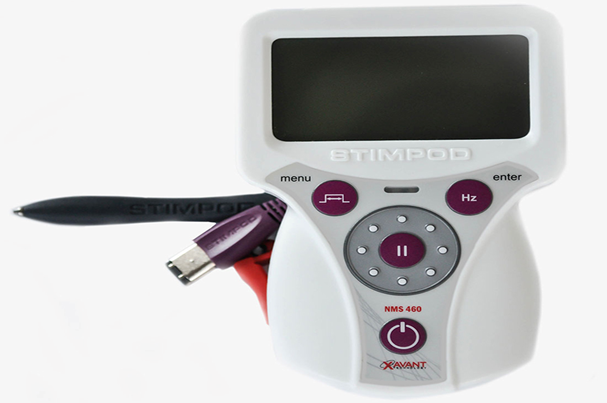
Xavant Technology Company, based in Pretoria, South Africa, has been approved by the FDA (Food and Drug Administration - US Food and Drug Administration) through the peripheral nerve stimulation system NMS 460. This device Used to address chronic pain, postoperative pain, acute post-traumatic pain, and pain control resulting from routine rehabilitation techniques.
This device delivers pulsed frequency waves (PRF - Pulsed Radio Frequency), through the skin. The company claims that the electromagnetic effects are similar to the devices nerve stimulating produces, offering some benefit of the device without natural invasions.
The device also has a nerve fiber distribution probe that helps detect damaged nerves and assesses their status and how they respond to treatment.
.
Source : www.medgadget.com
Translation: Dr. Nguyễn Hữu Tùng & partner
Other healthcare
- Development history of world medicine ( 11:44 - 13/10/2017 )
- Microfluidic channel device for tumor chemotherapy ( 11:40 - 12/10/2017 )
- Higher education in the era of Industrial Revolution 4.0 ( 11:37 - 08/11/2017 )
- DRG Nerve Stimulant Device ( 11:19 - 09/10/2017 )
- Surgeons view CT, MRI, ultrasound images using 3D technology ( 11:14 - 07/10/2017 )
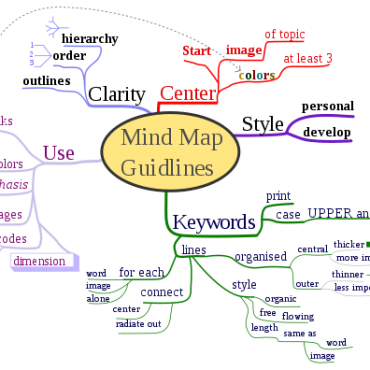
- Steps to make mind map (Mind map) ( 11:00 - 07/10/2017 )
- Mind mapping software ( 10:41 - 07/10/2017 )
- Mind map is what? ( 10:28 - 18/10/2017 )
- Schizophrenia is 80% hereditary ( 10:21 - 05/10/2017 )
- Ten ancient pain relievers ( 15:20 - 06/10/2017 )