Contact Admission
International Collaboration
Biological device for patients with end-stage renal failure
End-stage kidney failure is on the rise in Malaysia. Nationally, the number of patients with renal impairment will increase to 10,208 by 2020. Globally, 2.6 million patients with end-stage renal failure require dialysis by 2010 and this figure is estimated. calculation will almost double by 2030.
End-stage kidney failure occurs when both kidneys in the body are unable to function, producing urea toxin - the urea produced by the kidneys into the urine to accumulate in the body, other kidney functions such as keeping The salt and water in the body is also in trouble. When this happens, patients have to undergo dialysis to keep all these factors in control, which can be extremely uncomfortable and even fatal.
Globally, 2.6 million patients with end-stage renal failure required dialysis in 2010, and this number is estimated to nearly double by 2030.
Improve patient's life
Dialysis is time-consuming, money-consuming and does not benefit the patient. Moreover, the donor is limited, leaving many patients trapped in dialysis ulcers or facing death. due to non-dialysis.
Therefore, scientists at the University of California, San Francisco are currently working on a kidney biometric device that performs more than part of the kidney function, freeing the patient from the dialysis ring.
Hemodialysis machine
Kidney Biology Device consists of 2 parts:
The efficient membranes are created using nanotechnology silicon that filters toxins from the blood without the need for pumps or an external power supply.
Bioreactors contain biological kidney cells that perform other kidney functions such as regulating salt and water in the body.
This biological device will be implanted into the body through surgery similar to kidney surgery, under general anesthesia. The device is connected to the body's blood circulation system and will filter out toxins as waste is removed from the body through the bladder, just like what a kidney does.
This is great because the patient will no longer need to be dependent
dialysis machine and can eat and drink more normally.
When is this device ready?
The team is expected to begin clinical trials of the device in early 2018. During that time, they will determine whether the components of the device are safe for humans. This is one of the many steps for them to gain FDA (Food and Drug Administration) approval to keep up with other tests to confirm effectiveness, ability and safety. full of kidney biological equipment.
They hope to reach the final stage of clinical trials (the pre-public phase) by 2020.
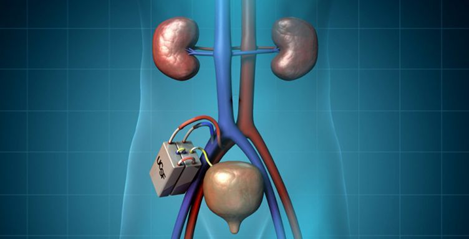
Image source: https://pharm.ucsf.edu/kidney
Side effects and risks
Preliminary tests have shown that the renal bioformation does not cause rejection, so patients on renal bioformation do not need immunosuppressants, unlike in patients receiving kidney transplantation. Furthermore, it also doesn't cause blood clots, unlike certain heart transplants, so blood-thinning drugs are also not necessary.
Putting this biologic in into the body carries the same risk as kidney transplant surgery. Risk of infection, surgery failure, trauma and scarring.
This study is sure to be interesting and will be full of predictions as it could change the lives of many end-stage kidney failure patients.
Efficiency and cost
This will be confirmed when the preclinical study is conducted, but for now, the researchers estimate that the glomerular filtration rate with the implanted device is about 20-30 ml / min.
The cost of treatment with a kidney bioform is estimated to be similar to that of a normal kidney transplant. This study is sure to be interesting and predictable as it could change the lives of many end-stage renal failure patients.
* GFR (Glomerular Filtration Rate) is the glomerular filtration rate, so it is a way to measure the effectiveness of the kidneys. The renal glomerular filtration rate is normally more than 90 ml / min, while patients with end-stage renal failure are less than 15 ml / min.
Source: https://dosinghealth.com/2018/03/29/implantable-bionic-kidney-the-holy-grail-for-patients-with-end-stage-renal-disease/
Translation summary: Dr. Nguyen Huu Tung et al
Other news
- How Dangerous Is Nipah Virus? Medical Alert and Urgent Health Recommendations ( 14:13 - 27/01/2026 )
- Predicting Disease from Sleep – A New Breakthrough Study ( 14:01 - 13/01/2026 )
- Medical advances predicted to break through in 2026 ( 13:54 - 12/01/2026 )
- Vietnamese medical miracles in 2025 – inspiration for medical students ( 07:54 - 07/01/2026 )
- Updating the SOFA-2 Score: A New Standard in the Assessment of Multiple Organ Failure After Three Decades ( 10:40 - 31/12/2025 )
- Home AEDs: High Life-Saving Effectiveness, but Not Cost-Effective at Current Prices ( 14:12 - 18/12/2025 )
- Artificial Intelligence and Pediatric Care ( 08:27 - 16/12/2025 )
- Applying Clinical Licensing Principles to Artificial Intelligence ( 09:36 - 08/12/2025 )
- U.S. Approves Targeted Lung Cancer Therapy Datroway ( 08:43 - 25/06/2025 )
- Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing ( 08:38 - 23/11/2023 )